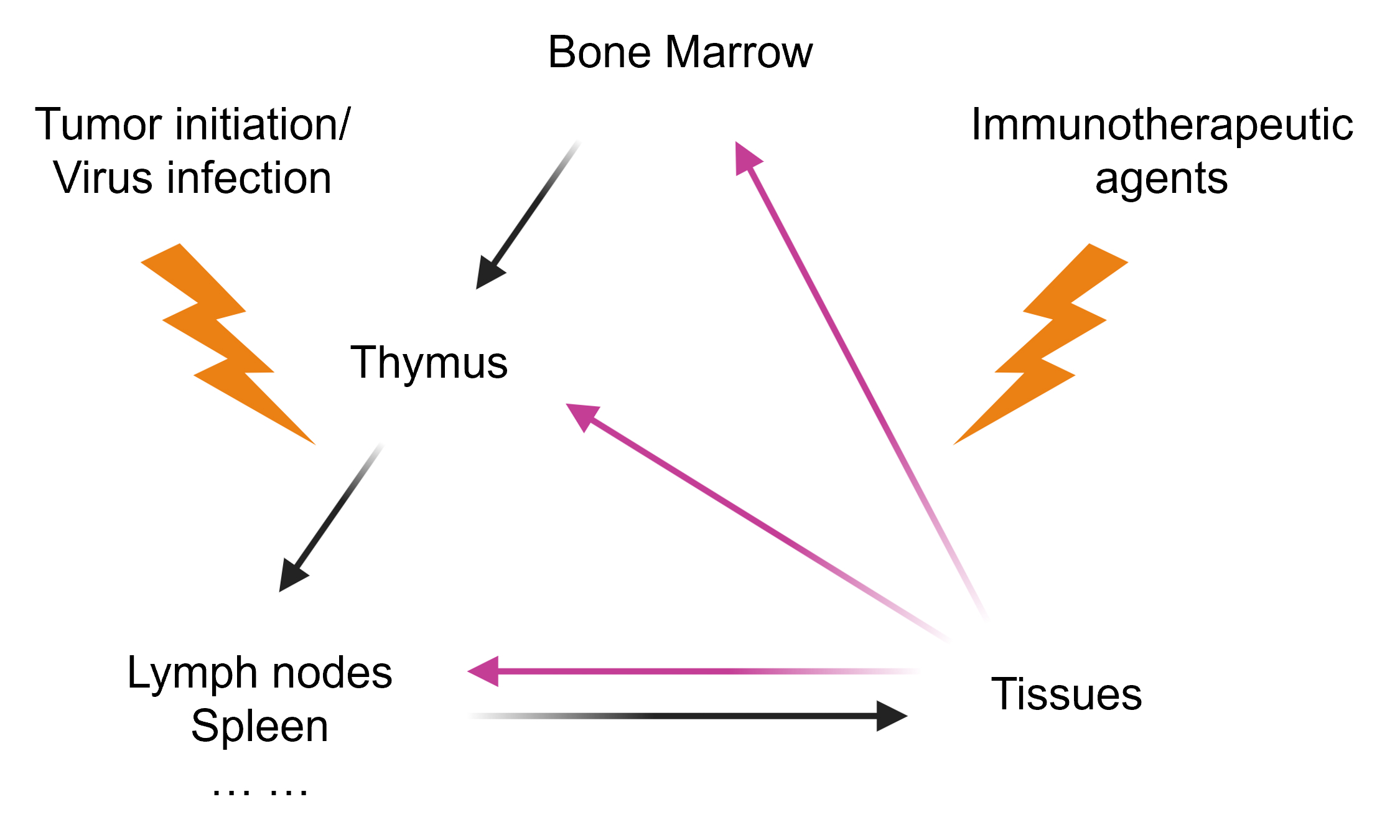
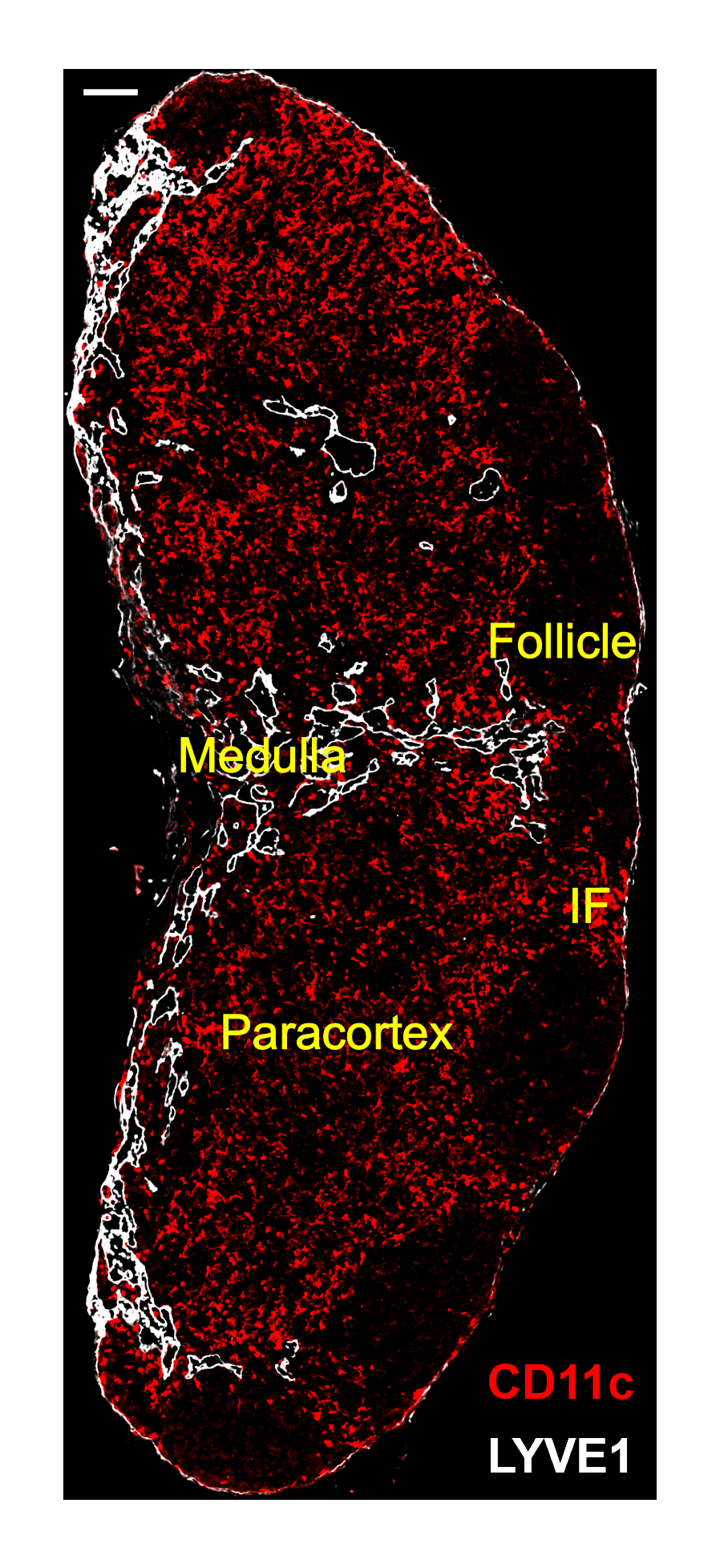
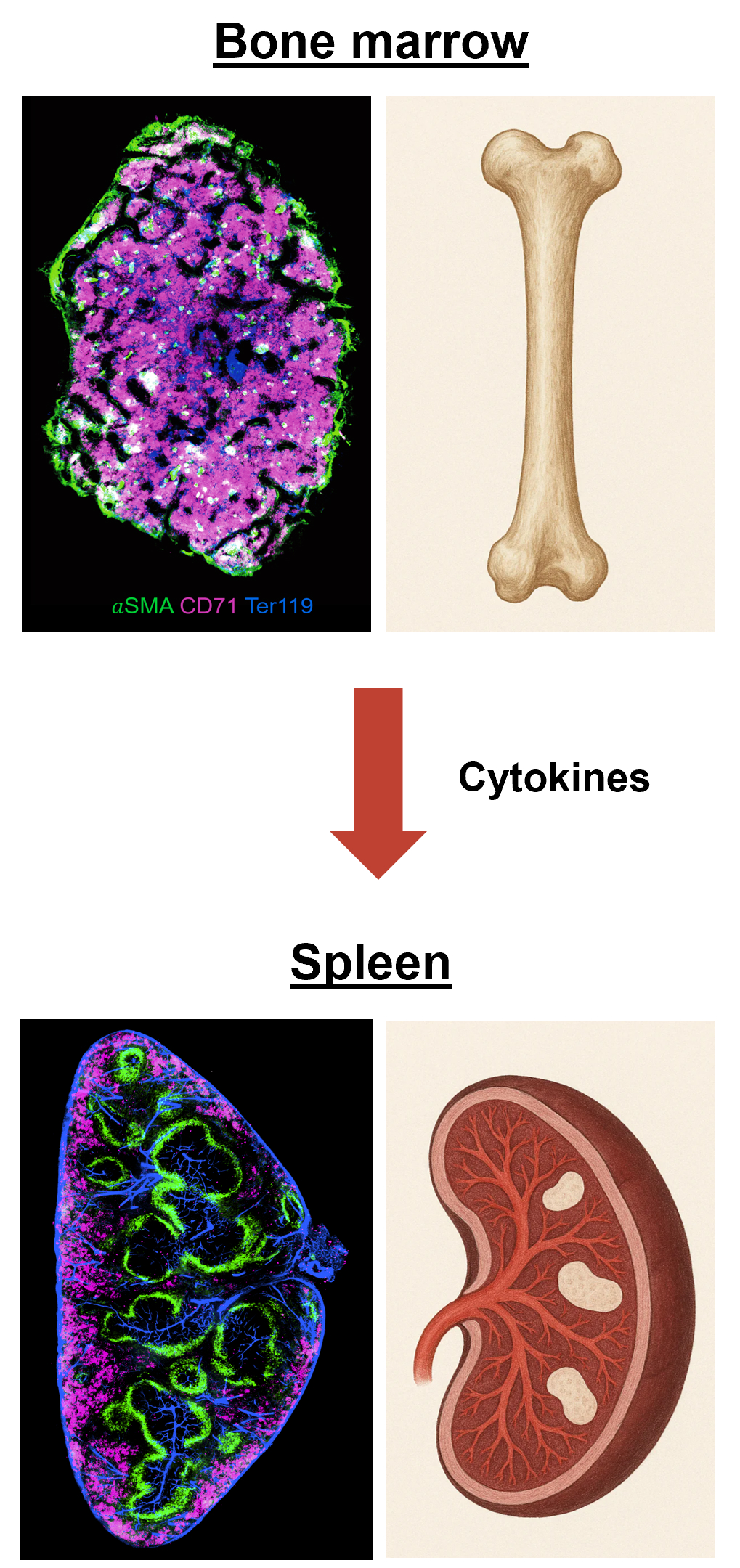
Our lab focuses on immune crosstalk between tissues and lymph organs. The unfolding of our research is guided by two principles. First, the generation of robust anti-tumor immunity requires coordinated events in the tumor microenvironment (TME) and in lymphoid organs, a concept often referred to as the “cancer-immunity cycle”. Tumor antigens released in the TME must reach the tumor draining lymph nodes (tdLNs) to prime naive T cells. Dendritic cells (DCs) play a central role in this cycle, which they not only present antigens to T cells in tdLNs, but also re-stimulate and expand effector T cells within the TME. Immune checkpoint blockade (ICB) therapies, such as anti-PD-1/PD-L1, function by unleashing DC-T inhibition at both sites. Therefore, crosstalk between the TME and tdLNs forms a self-propagating immunity loop that is critical for both endogenous anti-tumor responses and exogenous immunotherapies. Second, the immune system functions as a multi-organ network sustained by continuous immune cell development and trafficking. Central lymphoid organs like the bone marrow (BM) (and thymus for T cells) constantly generate new immune cells, which then populate peripheral tissues and secondary lymphoid organs (lymph nodes, spleen) to execute immune functions. Reciprocally, peripheral tissues provide instructive signals that feed back into lymphoid organs in both homeostasis and disease conditions (e.g., tumor growth or infection), and modulate immune cell development and activation in these immune organs. Thus, understanding the bidirectional interactions between tissues and lymphoid organs is fundamental to immunology.
Based on this perspective, our lab gradually stepped into two intertwined themes: (1) how pathological processes (tumor progression or infection in a tissue) alter the communication between tissues and lymphoid organs; and (2) how immunotherapeutic agents can induce systemic “butterfly effect” perturbations across multiple organs of the immune system. To address these themes, we have developed specific directions as detailed below.